Welcome to your ultimate resource for understanding stainless steel! Ever wondered what makes this remarkable material so strong, shiny, and resistant to rust? From the kitchen sink to towering skyscrapers, stainless steel is a true workhorse in our modern world.
In this comprehensive guide, we’ll unlock the secrets behind its “stainless” magic, explore its fascinating history, dive into its diverse types and grades, and discover why it’s the go-to choice for countless applications. Whether you’re a curious learner, a student, or a professional, get ready to explore everything you need to know about this incredibly versatile alloy!
Table of Contents
I. Introduction: Why is Stainless Steel So Important?
Ever wondered what makes that shiny kitchen sink so resilient, or how surgical tools stay so clean? Chances are, you’re thinking about stainless steel! This amazing material is everywhere – from towering skyscrapers to the tiny screws in your glasses. It’s more than just a pretty face; stainless steel is a powerhouse of strength, an champion of cleanliness, and a true marvel of modern science.
Think of it like this: stainless steel is the superhero of metals. It fights off rust and corrosion with a special invisible shield, stands up to tough conditions, and still manages to look good doing it. For decades, engineers, designers, and even everyday folks like us have relied on its unique qualities. Whether you’re a student curious about materials, a professional choosing the right metal for a project, or just someone who appreciates well-made things, understanding stainless steel is incredibly rewarding.
In this guide, we’re going on an adventure to uncover everything about stainless steel. We’ll explore what it’s made of, journey through its fascinating history, meet its different family members (types and grades!), and discover its incredible properties. We’ll also peek into how it’s made and see all the amazing ways it’s used in our world. So, get ready to become a stainless steel expert!

II. What Exactly IS Stainless Steel? Defining the Alloy
So, what’s the secret sauce that makes stainless steel, well, stainless? At its heart, stainless steel is a special kind of metal mix, called an alloy. Imagine making a cake – you mix flour, sugar, eggs, and other ingredients to get something new and delicious. Alloys are similar; they’re made by mixing a main metal with other elements to give it new and improved powers.
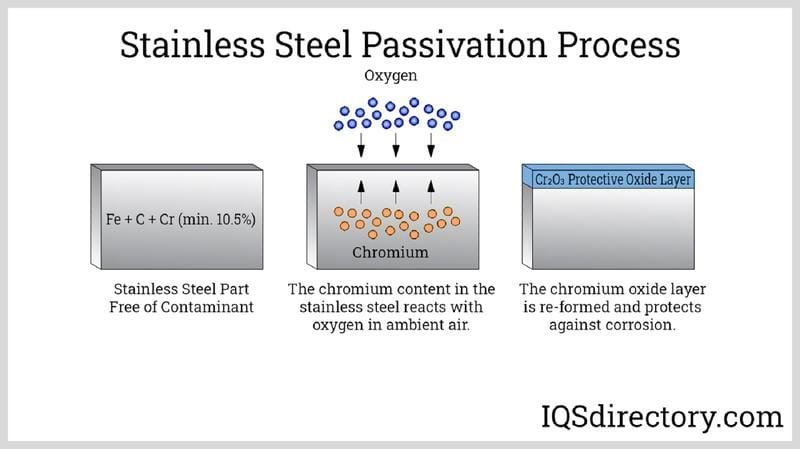
The main ingredient in stainless steel is iron, just like in regular steel. But here’s the magic part: to become “stainless,” iron needs a special helper – chromium. Lots of it! To officially be called stainless steel, the mix must have at least 10.5% to 11% chromium. This isn’t just a random number; this amount of chromium is the key to stainless steel’s superpower: its amazing resistance to rust and corrosion.
A. The “Stainless” Property: How Does Stainless Steel Resist Corrosion?
You might be wondering, “How does chromium stop rust?” It’s a clever trick of chemistry! When chromium is part of the stainless steel mix and comes into contact with oxygen (which is all around us in the air and water), it forms an incredibly thin, invisible, yet super tough layer on the surface of the steel. This layer is called the chromium oxide (Cr₂O₃) passive layer.
Think of this passive layer as an invisible suit of armor. It’s so thin you can’t see it, but it’s incredibly strong and sticks tightly to the steel. This armor protects the iron underneath from attacking elements like water and oxygen that cause rust. Here’s the really cool part:
- It’s Tenacious: This layer clings to the steel and doesn’t easily wear off.
- It’s Self-Healing: If the stainless steel gets scratched or damaged, and the passive layer is broken, don’t worry! As long as there’s chromium in the steel and oxygen around, the layer will quickly repair itself. It’s like a superhero regenerating its shield!
The more chromium in the steel, generally, the better this protective layer is, and the better the stainless steel can fight off corrosion. Other elements added to the mix can also boost this protection, especially in really tough environments. We’ll talk more about those later!
B. Is Stainless Steel a Compound?
This is a common question! While stainless steel has a very specific recipe, it’s not technically a chemical compound like water (H₂O) or salt (NaCl). Instead, as we’ve learned, stainless steel is an alloy. In simpler terms, it’s a solid mixture where the different metal atoms (iron, chromium, nickel, etc.) are all mixed together, but they don’t form new, distinct molecules in the way compounds do. Think of it more like a very well-mixed fruit salad rather than a smoothie where everything is chemically bonded.
C. Common Misconceptions about Stainless Steel
One of the biggest myths is that “stainless steel never rusts.” While it’s incredibly resistant, it’s not completely immune. In very harsh conditions – like constant exposure to saltwater, certain strong acids, or if it’s a lower-quality grade not suited for the job – even stainless steel can show some signs of corrosion. But, compared to regular steel, its ability to stay “stainless” is truly remarkable. The key is choosing the right type of stainless steel for the right job, something we’ll dive deep into!
III. The Fascinating History of Stainless Steel: A Century of Innovation
The story of stainless steel isn’t just about one “aha!” moment. It’s a tale of curious scientists, persistent inventors, and step-by-step discoveries spanning over a hundred years! Let’s take a trip back in time.
A. Early Discoveries and Observations (Pre-20th Century)
Long before stainless steel got its name, people were noticing something special about chromium, its star ingredient.
- Way back in 1798, a smart Frenchman named Louis Vauquelin was the first to identify chromium as an element.
- Throughout the 1800s, scientists like J. Stoddart, Michael Faraday (yes, the famous one!), and R. Mallet noticed that when chromium was mixed with iron, it seemed to resist rusting and oxidation much better than iron alone.
- In 1821, another Frenchman, Pierre Berthier, proved that these chromium-iron mixes were also good at resisting acids. This was a big clue!
- By the 1840s, clever steelmakers in Sheffield, England (a place famous for steel!), and a company called Krupp in Germany started producing some types of chromium steel.
- Robert Mushet, in 1861, even got a patent for his version of chromium steel, hinting at its use for strong things like bridges.
- As the century neared its end, around the 1890s, scientists figured out how to make chromium without too much carbon in it – a crucial step for making better quality stainless-like steels.
- Between 1904 and 1911, Leon Guillet in France was making alloys that were very, very close to what we now know as stainless steel. He was on the right track!
- People were even experimenting with it for big things. In 1908, a ship hull was built using a chrome-nickel steel, and in 1911, a patent was granted for an “austenitic” type of stainless steel (often called 18/8, which we’ll learn about).
So, you see, the stage was being set. Many brilliant minds were contributing pieces to the puzzle.
B. The “Invention” of Stainless Steel: Key Milestones and Figures
While many contributed, a few names stand out when we talk about the “birth” of stainless steel as we know it.
- Harry Brearley (Sheffield, UK) – 1913: This is often the name most associated with the invention. Brearley was a metallurgist working in Sheffield, trying to find a better steel for gun barrels that wouldn’t wear out so quickly. As he experimented, he noticed one of his samples, containing about 12.8% chromium, wasn’t rusting! Instead of corroding, it stayed shiny. He quickly realized this “rustless steel” (as he called it) could be perfect for cutlery. And that’s how the first stainless steel knives came to be!
- Elwood Haynes (USA) – 1919: Across the pond, in America, Elwood Haynes was also working with chromium alloys. In 1919, he received a patent for a type of martensitic stainless steel.
It’s like a scientific race where different people were crossing similar finish lines around the same time! Both Brearley and Haynes made huge contributions.

C. Early Industrial Adoption and Expansion (1919-1930s)
Once the secret was out, stainless steel started popping up in all sorts of places.
- The companies in Sheffield and others quickly started using it to make things like surgical scalpels (because they could be easily sterilized and wouldn’t rust), cutlery, and various tools.
- By 1926, doctors were even using it for surgical implants.
- In 1928, big fermenting vessels (like those used for making beer or chemicals) were made from stainless steel because it wouldn’t contaminate the product.
- A new type called “precipitation hardening” stainless steel was discovered by William Kroll in 1929.
- And in 1930, Avesta Ironworks in Sweden was the first to produce “duplex” stainless steel, another special variety.
- The 1930s saw even bigger things: the USA built its first stainless steel train! In 1931, it was used to make parts for aircraft. And by 1935, shiny kitchen sinks made from stainless steel started appearing in homes.
D. Mid-20th Century to Present Day: Continued Development and Applications
Stainless steel didn’t stop evolving. Throughout the 1950s and right up to today, scientists and engineers have been finding new ways to make it better and use it in more applications.
Just imagine:
- 1954: Stainless steel was used to make underwater TV cameras.
- 1966: Tough stainless steel turbine blades were spinning in engines.
- 1980: A mobile flood barrier was built using this trusty material.
- 2010: Even the drums in our washing machines got a stainless steel upgrade for durability!
New manufacturing methods, like Argon Oxygen Decarburization (AOD), made it easier to produce high-quality stainless steel with precise ingredients. Super strong “superalloys” and highly specialized grades were developed for the most demanding jobs, like in aerospace or deep-sea exploration. And even today, researchers are still tinkering, trying to improve its strength, corrosion resistance, and other properties. It’s a story that’s still being written!
E. Table Summary: Key Dates, People, and Milestones in Stainless Steel History
Here’s a quick look at some of the highlights in stainless steel’s journey:
| Timeframe/Date | Person/Company Responsible | Key Milestone in Stainless Steel History |
|---|---|---|
| 1798 | Louis Vauquelin | Identifies chromium. |
| 1821 | Pierre Berthier | Proved chromium is resistant to acids. |
| 1904-1911 | Leon Guillet | Produces stainless steel-like alloys. |
| 1913 | Harry Brearley | Accredited for inventing “rustless steel” with 12.8% chromium. |
| 1919 | Elwood Haynes | Got a patent for martensitic SS. |
| 1919-1930 | Sheffield and other companies | Used for surgical scalpels, cutlery; surgical implants (1926); fermenting vessels (1928). |
| 1929 | William Kroll | Discovered precipitation hardening stainless steel. |
| 1930 | Avesta Ironworks | First to produce duplex stainless steel. |
| 1930-1935 | – | First stainless steel train (USA); used for aircraft (1931); kitchen sinks (1935). |
| 1950s to date | – | Continuous development: underwater TV camera (1954), turbine blades (1966), mobile flood barrier (1980), washing machine drums (2010). |
This incredible journey of discovery and innovation has made stainless steel one of the most versatile and important materials in our modern world. Next, let’s look closer at what actually goes into making it!
IV. Deconstructing the Alloy: What is Stainless Steel Made Of? (In-Depth Composition)
We know iron and chromium are the main stars in stainless steel, but there’s a whole supporting cast of other elements that play crucial roles. Think of it like a master chef creating a signature dish – each ingredient, even in small amounts, contributes to the final flavor and texture. In stainless steel, these extra elements fine-tune its properties, making it suitable for a vast range of jobs. Let’s break down the recipe!
A. Primary Alloying Elements and Their Roles:
The exact mix varies depending on the “grade” or type of stainless steel we want to make, but here are the key players you’ll often find:
- Iron (Fe): The FoundationIron is the main component, making up the bulk of the alloy (often 70-80% or more). It provides the basic strength and structure, the metallic backbone upon which stainless steel is built.
- Chromium (Cr): The Protector – The Defining Element!This is the absolute hero ingredient. As we’ve learned, you need at least 10.5% chromium for steel to be “stainless.” Its main job is to form that amazing, thin, self-healing passive layer of chromium oxide on the surface. This layer is what shields the iron from rust and corrosion. Generally, the more chromium you add (some grades go up to 26-30%!), the stronger this shield becomes, and the better the steel can resist attack in harsh environments.
- Nickel (Ni): The Versatility EnhancerNickel is like the multitalented actor in our cast. It does a lot of important things:
- Promotes Austenitic Structure: Nickel helps to form and stabilize a particular crystal structure in the steel called “austenite” (we’ll talk more about this structure soon). Austenitic stainless steels are the most common type.
- Improves Formability and Ductility: It makes the steel easier to bend, shape, and draw into wires without breaking.
- Boosts Weldability: Steels with nickel are generally easier to weld.
- Enhances Toughness: Especially at very cold (cryogenic) temperatures, nickel helps keep the steel from becoming brittle.
- Increases Corrosion Resistance: It further improves resistance to many types of corrosion, especially in acidic conditions.
You’ll find nickel in amounts from around 8% (like in the popular 304 grade) up to 22%, or even as high as 35% in some special austenitic grades.
- Carbon (C): The Strength Giver (But Handle with Care!)Carbon is a classic strengthening element in all steels. A little bit can make stainless steel harder and stronger. However, in stainless steel, carbon can be a bit of a troublemaker if not managed carefully. If there’s too much carbon, and the steel is heated (like during welding), carbon can snatch chromium atoms to form “chromium carbides.” When this happens, there’s less chromium available to form that vital protective passive layer, making the steel vulnerable to a type of corrosion called “intergranular corrosion” or sensitization.
That’s why many modern stainless steels, especially those meant for welding, are “L” grades (like 304L or 316L), which means they have very low carbon content (e.g., less than 0.03%). However, some types of stainless steel, like martensitic grades used for knives, intentionally have higher carbon (up to 1.2%) to achieve extreme hardness. - Molybdenum (Mo): The Pitting FighterIf stainless steel is going to face off against chlorides (like in seawater, de-icing salts, or some industrial chemicals), molybdenum is its best friend. Adding just a few percent of molybdenum (often 2-3% in grade 316, and more in super austenitic and duplex grades) drastically improves resistance to “pitting corrosion” (tiny, deep holes) and “crevice corrosion” (corrosion in tight gaps). Molybdenum also helps to strengthen the steel at high temperatures.
- Manganese (Mn): The Helper Stabilizer and DeoxidizerManganese is another useful element. It helps to remove unwanted oxygen during the steelmaking process (acting as a deoxidizer). It also contributes to strength and hardness. In some stainless steel types (like the 200 series), manganese is used in larger amounts to help form the austenitic structure, partly replacing some of the more expensive nickel.
- Silicon (Si): Another Deoxidizer and Strength BoosterLike manganese, silicon helps remove oxygen during steelmaking. It also adds some strength. Importantly, silicon can improve the steel’s resistance to scaling (flaking off) at high temperatures and can boost its fight against very strong oxidizing acids, like concentrated nitric acid and sulfuric acid.
- Nitrogen (N): The Strength and Pitting Resistance ChampionNitrogen is a powerful player. It’s very effective at stabilizing the austenitic structure. More impressively, it significantly increases the steel’s yield strength (its resistance to starting to bend permanently). Nitrogen is also excellent at improving resistance to pitting corrosion, often working hand-in-hand with molybdenum. You’ll find it intentionally added to many duplex stainless steels and some high-nitrogen austenitic grades.
- Titanium (Ti) & Niobium (Nb) (also called Columbium): The Carbide TamersRemember how carbon can cause trouble by forming chromium carbides? Titanium and niobium are like peacemakers. They have a stronger attraction to carbon than chromium does. So, if they are added to the steel, they will grab the carbon first, forming titanium carbides or niobium carbides. This leaves the chromium free to do its main job of forming the protective passive layer. These elements are called “stabilizers” and are found in grades like 321 (titanium-stabilized) and 347 (niobium-stabilized), which are great for welded structures that will be exposed to high temperatures, as they prevent sensitization.
- Copper (Cu): The Specialist for Corrosion and HardeningCopper isn’t always a major player, but it has its special roles. It can improve resistance to certain acids, like sulfuric acid. In some grades, it enhances resistance to seawater corrosion. Copper is also a key ingredient in some “precipitation hardening” stainless steels, helping them achieve very high strength through heat treatment.
- Aluminum (Al), Vanadium (V), Tungsten (W): Other Special ModifiersThese elements, and others, can be added in small amounts for very specific purposes. Aluminum, for instance, is important in some precipitation hardening grades. Vanadium and tungsten can contribute to strength and performance at very high temperatures.
B. How Alloying Elements Influence Crystal Structure (Austenite, Ferrite, Martensite)
It’s fascinating how these ingredients don’t just change properties like corrosion resistance, but they also affect the very way the atoms arrange themselves in the steel! This atomic arrangement, or “crystal structure,” is fundamental to defining the main types of stainless steel.
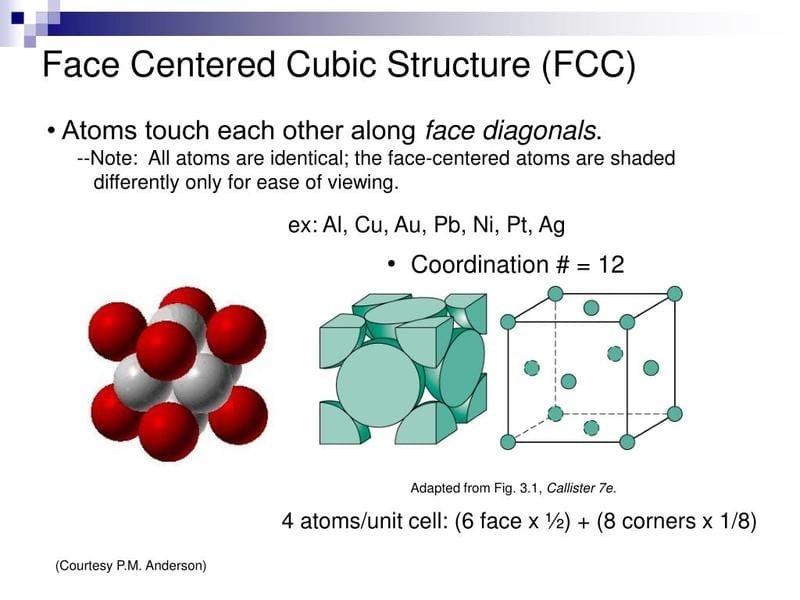
Some elements are “austenite formers” – they encourage the steel to have a face-centered cubic (FCC) atomic structure. Nickel is the most famous austenite former, but manganese and nitrogen also help.
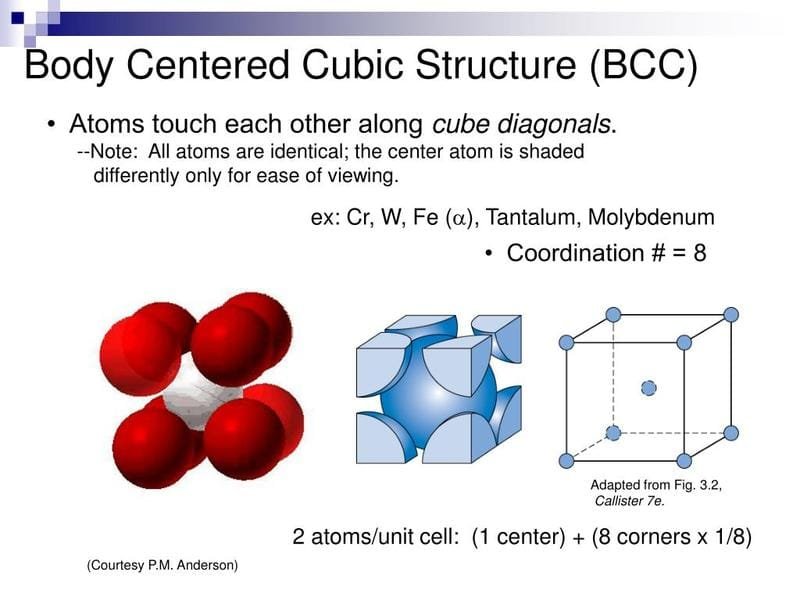
Other elements are “ferrite formers” – they promote a body-centered cubic (BCC) structure. Chromium is a strong ferrite former, and silicon, molybdenum, and titanium also push the steel towards this structure.
The balance between these austenite formers and ferrite formers, along with the carbon content and how the steel is heated and cooled, determines whether we end up with an austenitic, ferritic, martensitic, or even a mixed duplex stainless steel. Understanding this interplay is key to understanding the different “families” of stainless steel we’re about to explore!
V. The Families of Stainless Steel: Understanding the 5 Main Types
Now that we know what goes into stainless steel, let’s meet the family! Stainless steels aren’t all the same; they come in different “types” or “families,” each with its own personality and special talents. These types are mainly defined by their microscopic crystal structure, which, as we learned, is influenced by their chemical recipe and how they are made. There are five main families we should get to know:
- Austenitic
- Ferritic
- Martensitic
- Duplex (a mix of austenitic and ferritic)
- Precipitation Hardening (PH)
Let’s explore each one in detail!
A. 1. Austenitic Stainless Steel: The Workhorse
If stainless steel were a superhero team, austenitic stainless steel would be its most popular and versatile member. It’s the type you encounter most often, and for good reason!
- Microstructure: Austenitic stainless steels have a “Face-Centered Cubic” (FCC) crystal structure. Imagine a cube with an atom at each corner and one in the center of each face. This structure is key to many of its great properties.
- Key Characteristics:
- Excellent Corrosion Resistance: Generally, they offer fantastic protection against rust and many corrosive substances.
- Excellent Formability and Ductility: This means they are easy to bend, stretch, and shape without breaking. Think about how kitchen sinks are pressed into shape – that’s often austenitic stainless steel at work!
- Excellent Weldability: They are usually very easy to weld, which is great for making complex structures.
- Good Toughness: They are strong and resist fracturing, even at very, very cold (cryogenic) temperatures where other steels might become brittle.
- Generally Non-Magnetic: In their soft, annealed state, austenitic stainless steels are not magnetic. However, if you bend or work them a lot (cold working), they can become slightly magnetic.
- Not Hardenable by Heat Treatment: You can’t make them harder by heating and cooling them like some other steels. They get stronger and harder only when they are “cold worked” (shaped or bent at room temperature).
- Typical Composition: They are rich in chromium (usually 16% to 26%) and nickel (up to 35%, but commonly 8% to 22%). They also have low carbon content to maintain good weldability and corrosion resistance.
- Common Series/Grades:
- 300 Series: This is the most famous group, including the super-popular Type 304 (often called 18/8 because it has about 18% chromium and 8% nickel) and Type 316 (which has added molybdenum for even better corrosion resistance, especially against salts). Other members include 301, 302, 303, 309, 310, 321, and 347.
- 200 Series: These are also austenitic but use less nickel (which is expensive) and more manganese and nitrogen to achieve the austenitic structure. They offer a more economical option for some applications but might not have the same top-tier corrosion resistance as the 300 series in all environments.
- Applications: Their versatility makes them superstars in many fields! You’ll find them in:
- Food Processing & Kitchenware: Pots, pans, cutlery, sinks, commercial kitchen equipment, brewery tanks. (Why? Hygienic, easy to clean, doesn’t react with food, resists food acids).
- Chemical & Pharmaceutical Industries: Tanks, pipes, valves, reactors. (Why? Excellent resistance to many chemicals).
- Architectural Uses: Building facades, railings, decorative trim. (Why? Looks good, lasts long, resists weather).
- Medical Devices: Surgical tools, implants (some grades). (Why? Can be sterilized, biocompatible, corrosion-resistant).
- Aerospace Components: Some non-structural parts, fluid lines. (Why? Good strength-to-weight, good at low temps).
Think of austenitic stainless steel as your friendly, reliable, all-around performer. For example, the 304 grade is so common, it’s probably in your kitchen right now! If you need something tougher against salty environments, like near the ocean or for boat parts, then 316 grade, with its added molybdenum, is the hero you’d call upon.
B. 2. Ferritic Stainless Steel: The Magnetic and Economical Choice
Next up is ferritic stainless steel. This type has its own set of strengths and is often a more budget-friendly option, especially when you don’t need the absolute top-tier performance of austenitics.
- Microstructure: Ferritic stainless steels have a “Body-Centered Cubic” (BCC) crystal structure. Picture a cube with an atom at each corner and one single atom right in the very center of the cube. This structure makes them magnetic!
- Key Characteristics:
- Good Corrosion Resistance: They offer decent protection against rust, especially in milder environments. While generally not as corrosion-resistant as most austenitics, they can be very good for specific applications.
- Magnetic: Yes, these guys will stick to a magnet! This is a key way to tell them apart from most austenitic grades.
- Moderate Formability and Weldability: They can be shaped and welded, but perhaps not as easily or as extensively as austenitics.
- Cannot Be Hardened by Heat Treatment: Like austenitics, you can’t make them harder by simple heating and cooling. They can be moderately hardened by cold working.
- Good Ductility: They can be stretched and bent reasonably well.
- Good Resistance to Stress Corrosion Cracking (SCC): This is a big advantage! SCC is a type of corrosion that can happen when a material is under stress in a corrosive environment (especially one with chlorides). Ferritics are much better at resisting this than standard austenitics.
- Typical Composition: These steels are high in chromium (ranging from 10.5% up to 30%, but often in the 10.5% to 27% range). The key difference from austenitics is that they have very little or no nickel. Their carbon content is also kept low (e.g., less than 0.2%) to maintain their properties.
- Common Series/Grades: They mainly belong to the 400 Series. Common examples include:
- Type 430: A very popular general-purpose ferritic. It’s used a lot in decorative applications and appliances.
- Type 409: Often found in automotive exhaust systems due to its good high-temperature corrosion resistance and lower cost.
- Others include 434, 439, 441, and 446 (which has very high chromium for excellent scaling resistance at high temperatures).
- Applications: Because they are more economical (thanks to low/no nickel) and have good specific properties, ferritics are great for:
- Automotive Exhaust Systems: Mufflers, pipes. (Why? Good heat resistance, resists corrosion from exhaust gases, cost-effective).
- Appliances: Dishwasher linings, refrigerator panels, washing machine tubs. (Why? Good corrosion resistance in mild environments, magnetic properties can be useful, cost).
- Kitchenware: Some types of cookware (especially bases for induction cooktops due to magnetism), utensils. (Why? Good heat transfer, cost-effective).
- Architectural Trim & Decorative Applications: (Why? Good appearance, decent corrosion resistance).
- Industrial Equipment: For less aggressive chemical environments.
So, if you need a stainless steel that’s magnetic, offers good everyday corrosion resistance, is kind to your wallet, and is particularly good at resisting stress corrosion cracking, ferritic might be your go-to. For instance, Type 430 is often used for the shiny trim on appliances or for everyday cutlery. The inside of your dishwasher might very well be a ferritic grade!
C. 3. Martensitic Stainless Steel: Hardness and Strength
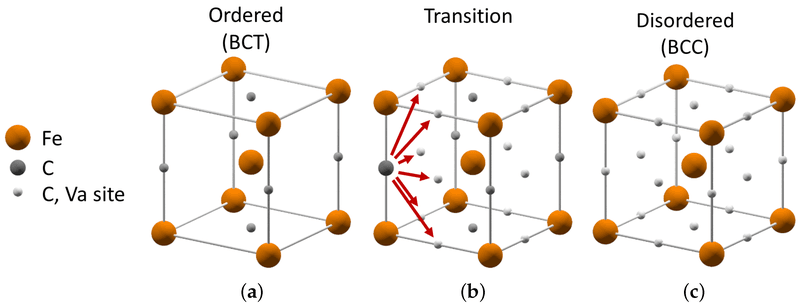
If you need stainless steel that’s incredibly hard and strong, something that can hold a sharp edge or withstand a lot of wear and tear, then you’re looking for martensitic stainless steel. These are the tough guys of the stainless world.
- Microstructure: Martensitic stainless steels get their unique properties from a “Body-Centered Tetragonal” (BCT) crystal structure. This special structure is formed when austenitic steel (with the right amount of carbon) is heated up and then cooled down very quickly (a process called quenching). It’s this transformation that makes them so hard.
- Key Characteristics:
- High Strength and Hardness: This is their superpower! They can be made very hard, much harder than austenitic or ferritic types.
- Hardenable by Heat Treatment: Unlike the previous two types, martensitic stainless steels can be significantly hardened by heating them and then rapidly cooling (quenching), followed by a “tempering” heat treatment to adjust the final hardness and toughness.
- Magnetic: Yes, these are also magnetic, like ferritics.
- Moderate Corrosion Resistance: Because they often have a bit less chromium and more carbon (which can tie up some chromium), their corrosion resistance is generally not as good as austenitic or ferritic steels. It’s a trade-off for their incredible hardness. However, it’s still much better than regular carbon steel!
- Limited Weldability: Welding martensitic steels can be tricky because of their high hardness and the risk of cracking. Special procedures are often needed.
- Typical Composition: They usually have a medium to high amount of chromium (typically 11% to 18%, often 11.5% to 18%), but the key difference is their higher carbon content (can be up to about 1.2%). Some may also contain a little nickel to improve properties.
- Common Series/Grades: These also fall into the 400 Series, but they are distinct from the ferritic 400s. Popular examples include:
- Type 410: A general-purpose hardenable stainless steel. Good for when you need decent strength and some corrosion resistance.
- Type 420: Has more carbon than 410, so it can be made even harder. This is a classic choice for cutlery and surgical instruments.
- Type 440 Series (440A, 440B, 440C): These have even higher carbon content, with 440C being one of the hardest stainless steels available. Excellent for high-quality knives, ball bearings, and valve parts that need to resist wear.
- Applications: Their hardness and strength make them ideal for:
- Cutlery: Knives, blades. (Why? Can be sharpened to a keen edge and hold it well).
- Surgical and Dental Instruments: Scalpels, forceps, drills. (Why? Hardness, can be sterilized, good wear resistance).
- Turbine Blades: For steam and gas turbines. (Why? High strength, can withstand high temperatures and stresses).
- Fasteners: Bolts, screws for demanding applications. (Why? High strength).
- Valves, Shafts, and Pump Parts: Where resistance to wear is important.
- Tools: Various hand tools, molds.
When you pick up a really good kitchen knife that stays sharp for a long time, there’s a good chance it’s made from a martensitic stainless steel like Type 420 or 440C. Their ability to be heat-treated to achieve high levels of hardness is what makes them perfect for such jobs.
D. 4. Duplex (Austenitic-Ferritic) Stainless Steel: The Best of Both Worlds?
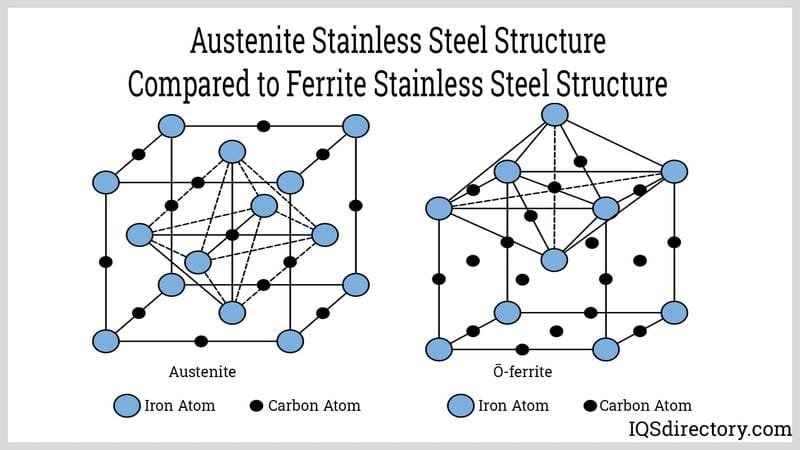
What if you could combine the best features of austenitic and ferritic stainless steels into one super-material? Well, that’s essentially what duplex stainless steels are! They are a fascinating family that offers a powerful combination of strength and corrosion resistance.
- Microstructure: As their name suggests, duplex stainless steels have a mixed microstructure. They are cleverly designed so that they have roughly equal amounts of austenite (FCC structure) and ferrite (BCC structure) – usually about 50/50. This balanced, two-phase structure is the secret to their enhanced properties.
- Key Characteristics:
- High Strength: Duplex steels are significantly stronger than standard austenitic grades like 304 or 316. Often, their yield strength (resistance to permanent bending) can be twice as high! This means you can often use less material to achieve the same strength, saving weight and cost.
- Excellent Corrosion Resistance: They generally have very good all-around corrosion resistance, often better than 316. They are particularly famous for their outstanding resistance to chloride stress corrosion cracking (SCC) – a major weakness of standard austenitic steels. They also have great resistance to pitting and crevice corrosion, especially the grades with higher chromium, molybdenum, and nitrogen.
- Good Weldability: While they need slightly different welding procedures than austenitics, duplex steels are generally considered to have good weldability, provided the right techniques and filler metals are used to maintain the balanced microstructure.
- Magnetic: Because they contain a significant amount of ferrite, duplex stainless steels are magnetic.
- Typical Composition: To achieve their dual-phase structure and excellent properties, duplex steels have a carefully balanced recipe. They typically contain:
- High chromium (usually 19% to 28%, often 21% to 27%).
- Moderate amount of nickel (usually 1.35% to 8%). Nickel helps form the austenite phase.
- Often, significant amounts of molybdenum (0.05% to 5%) for enhanced corrosion resistance.
- Nitrogen is also a very important addition; it helps form austenite, increases strength, and improves pitting resistance.
- Common Grades: Duplex steels are often categorized by their corrosion resistance or “PREN” (Pitting Resistance Equivalent Number – a number calculated from their composition that indicates pitting resistance).
- Lean Duplex: More economical grades with good strength and moderate corrosion resistance (e.g., 2304).
- Standard Duplex: The workhorse is Type 2205 (about 22% Cr, 5% Ni, 3% Mo). It offers a great balance of strength and corrosion resistance for many applications.
- Super Duplex: These have even higher amounts of chromium, molybdenum, and nitrogen for exceptional corrosion resistance in very aggressive environments (e.g., Type 2507, with about 25% Cr, 7% Ni, 4% Mo).
- Applications: Their unique combination of high strength and superb corrosion resistance makes them invaluable in demanding industries:
- Chemical Processing Industry: Tanks, pipes, heat exchangers for handling corrosive chemicals. (Why? Resists many chemicals and SCC).
- Oil and Gas Industry (Onshore & Offshore): Pipelines, valves, equipment for handling sour gas and seawater. (Why? High strength, resists corrosive oil/gas environments and SCC).
- Marine Environments: Shipbuilding, desalination plants, offshore platforms. (Why? Excellent resistance to seawater corrosion and SCC).
- Pulp and Paper Industry: Digesters, bleaching equipment. (Why? Resists corrosive chemicals used in paper making).
- Structural Components: Bridges, building supports where high strength and corrosion resistance are needed. (Why? Can use thinner sections due to high strength).
- Storage and Transportation: Aircraft storage containers, chemical shipping containers.
If a project demands serious strength combined with top-notch protection against nasty corrosive environments, especially those with chlorides, a duplex stainless steel like 2205 is often the champion. They allow engineers to design structures that are both lighter and more durable in tough conditions.
E. 5. Precipitation Hardening (PH) Stainless Steel: Ultra-High Strength
Last but certainly not least, we have precipitation hardening (PH) stainless steels. These are the true strongmen of the stainless world, capable of achieving incredible strength levels through a special heat treatment process, all while maintaining good corrosion resistance.
- Microstructure: PH stainless steels typically start with a martensitic or a mixed “semi-austenitic” microstructure. The magic happens during a heat treatment process called “aging.” During aging, tiny, hard particles “precipitate” (form) within the steel’s structure, acting like microscopic reinforcements that make the steel extremely strong.
- Key Characteristics:
- Very High Strength: This is their defining feature. They can be heat-treated to achieve some of the highest strengths available among all stainless steels.
- Good Corrosion Resistance: Depending on the specific grade, their corrosion resistance can be quite good, sometimes comparable to standard austenitic grades like 304.
- Good Toughness: Even at high strength levels, many PH grades maintain good toughness, meaning they can absorb energy before fracturing.
- Hardenable by Heat Treatment (Aging): They are supplied in a softer, “solution-treated” condition, which makes them easier to machine or form. Then, a relatively simple, low-temperature aging heat treatment is applied to develop their full strength.
- Magnetic: They are usually magnetic.
- Typical Composition: PH steels have a base of chromium (often 15% to 17.5%) and nickel (around 3% to 5%). The key to their hardening ability lies in the addition of small amounts of other elements that form those strengthening precipitates, such as:
- Copper (Cu)
- Aluminum (Al)
- Titanium (Ti)
- Niobium (Nb)
These hardening elements usually make up less than 0.5% of the steel’s total mass for elements like Al, Cu, and Nb.
- Common Grades: Some of the most well-known PH stainless steels include:
- 17-4 PH: (About 17% Cr, 4% Ni, plus copper and niobium). This is probably the most widely used PH grade. It offers an excellent combination of high strength, good corrosion resistance, and good toughness.
- 15-5 PH: Similar to 17-4 PH but often offers improved toughness and transverse (sideways) properties.
- 17-7 PH: (About 17% Cr, 7% Ni, plus aluminum). This grade can achieve very high strength and is often used for springs and other components requiring elasticity.
- Applications: Their ultra-high strength combined with good corrosion resistance makes them essential for critical, high-performance applications:
- Aerospace Industry: Aircraft structural components, landing gear parts, engine components, fasteners. (Why? Excellent strength-to-weight ratio, reliability).
- Shafts, Gears, and Valves: For demanding industrial machinery. (Why? High strength, wear resistance).
- Spindles: For high-speed rotating parts.
- High-Performance Engineering Components: Where extreme strength and reliability are paramount.
- Some military applications.
When engineers need a stainless steel that can handle enormous stresses and still resist corrosion, they often turn to PH grades like 17-4 PH. Their ability to be machined in a softer state and then hardened to achieve impressive strength makes them incredibly valuable for complex, high-performance parts.
F. Comparison Table: Key Properties of the 5 Stainless Steel Types
To help summarize, here’s a quick comparison of these five fascinating families of stainless steel:
| Property | Austenitic | Ferritic | Martensitic | Duplex | Precipitation Hardening (PH) |
|---|---|---|---|---|---|
| Corrosion Resistance | Excellent | Good | Moderate | Excellent (esp. SCC) | Good to Very Good |
| Strength | Moderate | Moderate | High to Very High | Very High | Very High to Ultra High |
| Ductility/Formability | Excellent | Good | Fair to Poor | Good | Fair to Good (in solution treated state) |
| Weldability | Excellent | Fair to Good | Poor to Fair (needs care) | Good (needs care) | Good (often before aging) |
| Magnetic? | Generally No (unless cold worked) | Yes | Yes | Yes | Yes (usually) |
| Hardenable by Heat Treatment? | No (only by cold work) | No (moderately by cold work) | Yes (quench & temper) | No | Yes (aging treatment) |
| Common Examples | 304, 316, 201 | 430, 409 | 410, 420, 440C | 2205, 2507 | 17-4 PH, 15-5 PH |
Understanding these five main types is the first big step to choosing the right stainless steel. Next, we’ll zoom in even further and look at specific “grades” within these families!
VI. Cracking the Code: Stainless Steel Grades Explained
We’ve met the five main families of stainless steel. Now, let’s get a bit more specific and talk about “grades.” You might hear people talking about “304 stainless” or “410 grade.” What does that mean? Think of it this way: if austenitic, ferritic, etc., are the broad family names, then grades are like the individual members of those families, each with their own unique characteristics and talents!
A. What is the Difference Between Stainless Steel “Types” and “Grades”?
It’s a subtle but important distinction:
- Types (or Families): As we just learned, these are based on the steel’s fundamental microscopic crystal structure (like austenitic, ferritic, martensitic, duplex, PH). This structure dictates broad characteristics like whether it’s magnetic or how it can be hardened.
- Grades: Within each type, there are many different grades. A grade refers to a specific chemical composition – the exact recipe of chromium, nickel, carbon, molybdenum, and other elements. This precise recipe gives that particular grade its unique set of properties, like its level of corrosion resistance, its strength, how easy it is to weld, or what applications it’s best suited for.
So, for example, 304 and 316 are both types of austenitic stainless steel, but they are different grades because 316 has molybdenum added to its recipe, giving it better corrosion resistance than 304 in certain environments.
B. Common Grading Systems: Speaking the Language of Steel
To keep all these different grades organized and ensure everyone around the world is talking about the same thing, various standard organizations have developed grading systems. You might come across a few different ones:
- AISI/SAE (American Iron and Steel Institute / Society of Automotive Engineers): This is probably the most widely recognized system, especially in North America. It uses three-digit numbers (like 304, 410, 201) to designate many common grades.
- ASTM (American Society for Testing and Materials): ASTM doesn’t usually assign the short grade numbers themselves, but they publish detailed specifications for stainless steels. These specifications define the required chemical composition, mechanical properties, testing methods, and more for various grades and product forms (like sheets, bars, pipes). For example, ASTM A240 is a common specification for stainless steel plates, sheets, and strips.
- UNS (Unified Numbering System): This system provides a more precise way to identify metals and alloys. A UNS number for stainless steel typically starts with an “S” followed by five digits (e.g., S30400 for Type 304, S31603 for 316L). It’s often used in conjunction with ASTM standards.
- EN (European Norm): This is the standard system used in Europe. It uses a numerical designation (like 1.4301, which is the EN equivalent of 304) and sometimes also a name designation.
- JIS (Japanese Industrial Standards): Japan has its own system. For example, SUS304 is the JIS equivalent of AISI 304.
While it might seem like a lot of codes, often there are direct equivalents between the systems. For everyday purposes, the AISI three-digit numbers are very commonly used.
C. Detailed Look at Common Stainless Steel Grades
Let’s dive into some of the most frequently encountered stainless steel grades, looking at their properties and where you’re likely to find them. As someone who has worked with these materials for years, I’ve seen firsthand how choosing the right grade can make or break a project. The details matter!
Austenitic Grades: The Versatile Performers
These are generally known for great corrosion resistance and formability. The 300 series is the most common, but the 200 series offers a more economical alternative in some cases.
- 200 Series (e.g., 201, 202, 205):
- 201: This grade has lower nickel content (manganese and nitrogen are used to help form the austenite structure instead). It’s a more affordable option suitable for things like flatware and some decorative applications. Its corrosion resistance isn’t as high as the 300 series, especially in harsh environments.
- 202: Similar to 201, also with lower nickel. Often used for making kitchenware.
- 205: Known for being good for “spin forming” (a metalworking process) and has low work hardening (meaning it doesn’t get super hard, super fast when you bend or shape it).
- 300 Series (The Stars of the Show!):
- 301: This grade work hardens quite rapidly, making it strong and suitable for applications like fasteners, springs, and trailer bodies.
- 302: A good, general-purpose austenitic stainless steel, very similar to 304 but with slightly higher carbon. Historically, it was more common before 304 became the standard.
- 303 (and 303Se): If you need to machine stainless steel easily, 303 is your friend! It has added sulfur (or selenium in 303Se) which makes it “free-machining.” This means it forms small chips when cut, making it much easier to work with on lathes and milling machines. Perfect for screw machine products, shafts, and fittings. The trade-off is slightly reduced corrosion resistance and weldability compared to 304.
- 304 (The “18/8” King!): This is arguably the most widely used stainless steel grade in the world. It contains roughly 18% chromium and 8% nickel (hence the nickname “18/8”).
“In my experience, 304 is the go-to for a huge range of applications due to its excellent balance of corrosion resistance, formability, weldability, and cost. It’s a true workhorse.”
It’s generally weldable and has good resistance to atmospheric corrosion and many chemicals. It’s an economical choice with low carbon content. However, it’s not great for seawater or chloride environments as it can suffer from pitting. You’ll find it in kitchen equipment, food processing machinery, architectural panels, tanks, and pipes.
- 304L (Low Carbon): The “L” stands for Low Carbon (typically ≤ 0.03%). This is super important! By keeping the carbon very low, 304L has excellent resistance to corrosion after welding because it minimizes the formation of those troublesome chromium carbides (sensitization). If you’re welding 304-type material, especially thicker sections, 304L is often the preferred choice.
- 305: Similar to 205, this grade is good for spin forming and has low work hardening. Used for parts that need significant shaping.
- 308: Often used as a filler metal for welding 304-type stainless steels. It offers good corrosion resistance and also good resistance to heat during the welding process.
- 309/309S: These grades have higher chromium and nickel content than 304, giving them better resistance to high temperatures and scaling (flaking). They are a perfect choice for making heat exchangers, furnace parts, and kiln linings. The “S” in 309S indicates a lower carbon version for improved weldability.
- 310/310S: Even more chromium and nickel than 309! These are serious high-temperature heroes, suitable for making furnaces, burners, and other components that see extreme heat. They are very resistant to scaling. Again, 310S is the low carbon version.
- 314: Similar to 310 but with added silicon for even better resistance to scaling at high temperatures. Best for making things like radiant tubes in furnaces.
- 316 (The “Marine Grade” Challenger!): This is the other superstar austenitic grade. The key difference from 304 is the addition of molybdenum (usually 2-3%). This molybdenum significantly boosts its resistance to pitting and crevice corrosion, especially in environments containing chlorides (like seawater, de-icing salts, and many industrial chemicals). If 304 isn’t tough enough for the corrosive conditions, 316 is often the next step up. Ideal for marine applications, chemical processing equipment, medical implants, and pharmaceutical equipment.
- 316L (Low Carbon Marine Grade): Just like 304L, the “L” means Low Carbon. This gives 316L excellent corrosion resistance after welding, making it the preferred choice for welded structures in corrosive environments. It’s probably the most specified grade for demanding applications where both corrosion resistance and weldability are critical.
- 317/317L: These have even more molybdenum than 316 (typically 3-4%), giving them even better resistance to pitting and crevice corrosion. They also have excellent “creep resistance” (resistance to slow deformation under stress at high temperatures). Used in very severe corrosive environments like pulp and paper mills. 317L is the low carbon version.
- 321: This grade is essentially 304 but with titanium added as a stabilizer. The titanium grabs any carbon, preventing chromium carbide formation during welding or high-temperature exposure. This means it retains its corrosion resistance even after being heated into the sensitization range (around 425-815°C or 800-1500°F). Good for applications with intermittent heating, like aircraft exhaust manifolds and expansion joints.
- 330: This is a high-nickel, high-chromium austenitic alloy designed for excellent resistance to oxidation, carburization (absorption of carbon at high temps), and thermal shock (rapid temperature changes). Often used for heat exchanger fixtures, furnace components, and baskets for heat treating.
- 347: Similar to 321, but it uses niobium (columbium) as a stabilizer instead of titanium. It also offers good resistance to intergranular corrosion after welding or high-temperature exposure. It has high “creep strength” (resists slow deformation under load at high temperatures), making it perfect for jet engine parts, heavy welded equipment, and high-temperature chemical process equipment.
- 348: A modification of 347, with restricted tantalum and cobalt content, making it suitable for nuclear service applications. It’s a low “retentivity” stainless steel grade (meaning it doesn’t hold onto magnetism easily).
- 384: This grade has higher nickel than 304, which gives it very low work hardening rates. This makes it suitable for severe cold forming operations, like making screws, bolts, and rivets that need a lot of shaping.
Austenitic-Ferritic (Duplex) Grades: The Strong Hybrids

As we learned, these combine the best of austenitics and ferritics.
- 329: An older duplex grade, it offers improved resistance to stress corrosion cracking and general corrosion compared to standard austenitics. Modern duplex grades like 2205 have largely superseded it due to even better properties.
- (Note: Specific grades like 2205 and 2507 were covered in Section V-D. They are very important duplex grades known for their high strength and excellent resistance to chloride SCC and pitting.)
Martensitic Grades: The Hard and Strong Contenders
These are all about hardness and strength, often used for cutting tools and wear-resistant parts.
- 403: A quality martensitic steel often used for highly stressed parts like steam turbine blades and compressor blades. It’s essentially a controlled-chemistry version of 410.
- 409: While listed here in one source, 409 is more commonly classified as a ferritic stainless steel. It’s known for good high-temperature corrosion resistance and is widely used for automotive exhaust systems. It generally does not require heat treatment for its applications. It’s a good general-purpose, cost-effective choice for construction where some corrosion resistance is needed.
- 410: This is the basic, general-purpose hardenable martensitic stainless steel. It has reasonable strength and corrosion resistance (to mild atmospheres, fresh water, some chemicals). It can be hardened by heat treatment. You can make machine parts, including shafts and exhausts (some types), pump parts, and bolts from it. It’s perfect for most general-purpose applications requiring moderate corrosion resistance and high strength.
- 414: This grade has nickel added (around 2%) to 410-type chemistry, which gives it better hardenability (it can be hardened more deeply and uniformly) and improved impact strength. You can use this grade to make springs and highly stressed parts.
- 416 (and 416Se): Like 303 is to 304, 416 is the free-machining version of 410. It has added sulfur (or selenium in 416Se) to make it much easier to machine. It’s the best for free machining among all martensitic grades. Used for shafts, gears, and other machined parts. Corrosion resistance is slightly lower than 410.
- 420: This grade has higher carbon content than 410, allowing it to be hardened to a greater degree. It’s widely used for cutlery, surgical instruments (like scalpels), needle valves, and shear blades. When polished, it has good corrosion resistance to mild atmospheres and fresh water.
- 422: A modification of 420, designed for high-temperature service up to 1200°F (650°C). It has good strength at elevated temperatures and is often used for making turbine blades, bolts, and valves in steam and gas turbines.
- 431: This grade has higher chromium and some nickel, giving it better corrosion resistance than 410 or 420, approaching that of some austenitic grades. It’s hardenable to high strength levels. Best for special-purpose applications like aircraft fasteners, shafts, and fittings requiring a combination of strength and corrosion resistance.
- 440A, 440B, and 440C: These are high-carbon, high-chromium martensitic stainless steels. They can achieve the highest hardness of all stainless steel grades after heat treatment, with 440C being the hardest. This makes them perfect for making bearing balls and races, high-quality cutlery, valve components, and wear-resistant parts. Corrosion resistance is moderate.
- 501: This is actually a heat-resistant chromium steel, sometimes considered a low-alloy steel rather than a true stainless by some classifications due to lower chromium (around 5%). It’s popular in making petrochemical equipment like refinery tubing due to its known high strength and excellent heat resistance at moderately high temperatures.
Ferritic Grades: The Magnetic and Economical Options
These are magnetic, generally more affordable, and offer good resistance to stress corrosion cracking.
- 405: This grade has a small amount of aluminum added, which prevents it from hardening when cooled from high temperatures (unlike 410). It’s non-hardenable by heat treatment and is often used for welded fabrications where 410 might crack.
- 409: (As mentioned under martensitics, often considered ferritic). It’s a low-cost, chromium stainless steel stabilized with titanium. It offers good atmospheric and exhaust gas corrosion resistance. It does not require heat treatment for its primary applications like automotive exhaust systems and some construction uses.
- 429: Similar to 405, but with slightly higher chromium. It has better welding properties than some other ferritics.
- 430: This is one of the most widely used ferritic stainless steels. It has good corrosion resistance to many mild environments and good formability. It’s non-hardening by heat treatment. You’ll find it in dishwashers (linings and baskets), automotive trim, refrigerator panels, and some kitchen equipment. It has a bright, attractive finish.
- 434: Similar to 430, but with added molybdenum for improved corrosion resistance, especially against de-icing salts used on roads. This makes it good for automotive trim in areas where road salt is common.
- 436: A modification of 434, with niobium (columbium) added for stabilization, which improves weldability and formability, especially for deep drawing. Also suitable for automotive trim and applications requiring good corrosion and heat resistance.
- 442: This grade has higher chromium content than 430, giving it better resistance to scaling at elevated temperatures. You can use it to make parts for furnaces and heaters. It has good high-temperature resistance.
- 446: With even higher chromium content (around 25%), this grade has excellent resistance to scaling and corrosion at very high temperatures. It’s one of the best ferritics for high-temperature service. You can use it to make pyrometer tubes (for measuring high temperatures), furnace parts, and burner components.
This list isn’t exhaustive – there are hundreds of grades! But these are some of the most common ones you’ll encounter. Choosing the perfect grade can seem daunting, but understanding their key characteristics is the first step. And of course, experts like us here are always ready to help guide you to the best material for your specific needs!
D. What do “L”, “H”, “N”, “S” suffixes mean in grade designations?

You’ll often see letters tacked onto the end of stainless steel grade numbers, like 304L or 310S. These aren’t random; they give us important clues about the steel’s specific properties:
- L (e.g., 304L, 316L): This almost always means “Low Carbon” (typically 0.03% carbon or less). Low carbon content is crucial for preventing sensitization (chromium carbide formation) during welding, which helps maintain the steel’s corrosion resistance in and around the weld. If you’re welding, “L” grades are often your best bet.
- H (e.g., 304H, 316H): This means “High Carbon” (typically 0.04% to 0.10% carbon). While low carbon is good for welding, a slightly higher, controlled carbon content can give the steel better strength at high temperatures (improved creep resistance). “H” grades are often used for elevated temperature service.
- N (e.g., 304N, 316N): This indicates that Nitrogen has been intentionally added to the steel. Nitrogen is a powerful strengthener in austenitic stainless steels and also improves pitting resistance.
- S (e.g., 309S, 310S): Often, “S” can also indicate a lower carbon version, similar to “L,” especially in older designations or for certain high-temperature grades. For example, 310S has lower carbon than standard 310 for better weldability and reduced carbide precipitation at high temperatures. (It’s important to check the specific standard, as “S” can sometimes have other meanings too, like indicating a silicon addition in some contexts, though less common for these specific grades).
These little letters can make a big difference in how the steel performs!
E. What is 18/8 Stainless Steel?
You’ll hear the term “18/8 stainless steel” a lot, especially when talking about kitchenware or food equipment. It’s a simple way of describing a very common type of austenitic stainless steel. The “18/8” refers to its typical chemical composition:
- 18% Chromium
- 8% Nickel
The most common grade that fits this description is Type 304 stainless steel. So, when someone says 18/8, they are usually referring to 304 or a very similar grade.
F. What is 18/10 Stainless Steel?
Similar to 18/8, “18/10 stainless steel” also refers to an austenitic grade with a specific composition:
- 18% Chromium
- 10% Nickel
The slightly higher nickel content (10% vs. 8%) in 18/10 generally gives it a bit better corrosion resistance and sometimes a brighter, more lustrous finish compared to 18/8. It’s often considered a slightly higher-end grade for cutlery and cookware. Grades like Type 304 (with higher end of Ni range) or even some versions of Type 316 can fall under the 18/10 description. The key is that it has that extra bit of nickel for enhanced performance and appeal.
Understanding these grades and designations empowers you to choose the right material for the job, ensuring performance, longevity, and safety. It’s a fascinating world once you start to decode the language of steel!
XIII. Applications Galore: Where is Stainless Steel Used? (Industry by Industry)
Stainless steel isn’t just a single material; it’s a vast family of alloys, each tailored for specific tasks. This versatility is why you find it in nearly every corner of our lives and industries. From the gleaming skyscrapers that pierce the clouds to the life-saving instruments in a surgeon’s hand, stainless steel plays a vital role. As someone who’s seen its application across numerous sectors, I’m always amazed by its adaptability. Let’s explore some of the key areas where stainless steel truly shines.
A. Architecture, Building, and Construction
The world of construction loves stainless steel for its blend of beauty, brawn, and longevity.
- Why Stainless Steel? Durability against weather, impressive strength for structural elements, and a sleek, modern aesthetic that architects adore. Plus, its corrosion resistance means less maintenance over the building’s life.
- Common Uses:
- Cladding and Facades: Giving buildings a stunning, contemporary look that lasts (e.g., the Chrysler Building’s iconic spire).
- Roofing: Long-lasting and resistant to harsh weather.
- Railings and Balustrades: Strong, safe, and visually appealing for stairs, balconies, and public spaces.
- Structural Supports: Beams, columns, and connectors in bridges and buildings, especially in corrosive environments like coastal areas. Duplex grades are often chosen here for their high strength.
- Monuments and Sculptures: For art that needs to endure the elements (e.g., The Cloud Gate “Bean” in Chicago).
- Window and Door Profiles: Offering strength and a clean look.
- Access Control Gates and Turnstiles: Durable for high-traffic public areas.

B. Automotive and Transportation
From your family car to massive freight trains, stainless steel keeps things moving safely and efficiently.
- Why Stainless Steel? Resistance to high temperatures (think exhaust systems!), corrosion from road salts and moisture, good strength-to-weight ratio, and it can be formed into complex shapes.
- Common Uses:
- Exhaust Systems: Mufflers, catalytic converters, pipes (often ferritic grades like 409 or austenitics like 304 for durability against heat and corrosive gases).
- Automotive Trim: For a shiny, durable finish on cars.
- Structural Components: In some vehicles for enhanced crash safety and longevity.
- Fuel Tanks: For corrosion resistance and safety.
- Springs and Blades: (Often martensitic grades for hardness).
- Fasteners: Throughout vehicles.
- Train Bodies and Components: Especially for passenger cars, offering durability and a sleek appearance.
- Aircraft Components: Certain grades are used for specific parts requiring heat or corrosion resistance.
The list of applications is truly staggering. Its unique combination of properties means that wherever there’s a need for durability, hygiene, corrosion resistance, or a material that can handle extreme conditions, stainless steel is often the top choice. I’ve seen it solve so many engineering challenges over the years – it’s a material that truly earns its keep.
Quick Overview of Stainless Steel in Key Industries
| Industry | Primary Stainless Steel Benefits | Common Stainless Steel Types/Grades Used | Example Applications |
|---|---|---|---|
| Food & Beverage | Hygiene, Corrosion Resistance, Non-reactivity, Cleanability | Austenitic (304, 316L) | Tanks, Piping, Cookware, Cutlery, Processing Equipment |
| Medical & Pharmaceutical | Biocompatibility, Sterilizability, Corrosion Resistance | Austenitic (316L), Martensitic (420, 440C), PH Grades | Surgical Instruments, Implants, Hypodermic Needles, Drug Mfg. Equipment |
| Chemical & Petrochemical | Resistance to Aggressive Chemicals, High Temp/Pressure Performance | Austenitic (316L, 317L, 310S), Duplex (2205, 2507) | Reactors, Storage Tanks, Piping, Heat Exchangers |
| Architecture & Construction | Aesthetics, Durability, Corrosion Resistance, Structural Strength | Austenitic (304, 316), Ferritic (430), Duplex | Cladding, Roofing, Railings, Structural Supports, Bridges |
| Automotive | Heat Resistance, Corrosion Resistance (exhaust, road salt), Strength | Ferritic (409, 430), Austenitic (304) | Exhaust Systems, Trim, Fuel Tanks, Structural Components |
XX. Frequently Asked Questions (FAQs) About Stainless Steel
Stainless steel is a fascinating material, and naturally, people have a lot of questions about it! Here are answers to some of the most common queries I encounter:
- A. What fields are stainless steel most commonly used in?
Oh, it’s almost easier to list where it isn’t used! But some of the biggest players are:
- Food and Beverage: From your kitchen spoon to giant brewery tanks.
- Architecture and Construction: Think shiny skyscrapers, sturdy bridges, and even your kitchen sink.
- Automotive: Especially in exhaust systems.
- Medical and Pharmaceutical: For everything from scalpels to life-saving implants.
- Chemical and Petrochemical: Handling tough chemicals safely.
- Energy Production: In power plants of all kinds.
Its versatility is truly remarkable!
- B. How do you maintain stainless steel to keep it looking new?
Good news – it’s pretty low maintenance!
- Routine Cleaning: Usually, warm water and a mild soap or detergent with a soft cloth or sponge is all you need. Always wipe in the direction of any polish lines. Rinse well and dry to prevent water spots.
- Tougher Stains: For things like fingerprints, a glass cleaner can work. For more stubborn stuff, specialized stainless steel cleaners or a paste of baking soda and water can be effective. Avoid harsh abrasives like steel wool unless you know what you’re doing, as they can scratch!
- Key “Don’ts”: Avoid chlorine bleach, abrasive scouring powders, and scrubbing against the grain.
- C. What is the difference between stainless steel and carbon steel?
This is a big one! Here’s a quick comparison:
Feature Stainless Steel Carbon Steel Corrosion Resistance Excellent (due to chromium) Poor (rusts easily without protection) Chromium Content Minimum 10.5% Very low Appearance Often shiny, can be polished Dull, often painted or coated Cost (Initial) Generally higher Generally lower Strength Good to very high, depending on grade Can be very strong, depending on grade/treatment In short, stainless steel’s superpower is its rust resistance, while carbon steel is often chosen for its strength and lower cost when corrosion isn’t a major concern (or can be managed with coatings).
- D. How do you calculate stainless steel weight?
You can get a good estimate of stainless steel weight using a simple formula: Weight = Density × Volume.
- Find the Density: The density of stainless steel varies slightly by grade, but a common average is around 7.85 to 8.0 grams per cubic centimeter (g/cm³) or about 490 pounds per cubic foot (lbs/ft³). You can often find specific densities for particular grades from suppliers.
- Calculate the Volume: This depends on the shape.
- For a flat sheet or plate: Volume = Length × Width × Thickness. Make sure all your units are consistent!
- For other shapes (bars, tubes), you’ll use the appropriate geometric formulas for volume.
- Multiply: Once you have the density and volume in compatible units, multiply them together to get the weight.
Many suppliers also offer online weight calculators on their websites, which can be very handy! Just remember, these calculations usually provide an estimate.
- E. How can you check the current prices of stainless steel?
Stainless steel pricing isn’t like buying a loaf of bread with a fixed price tag. It fluctuates based on many factors:
- Grade: Some grades with more exotic alloying elements (like nickel or molybdenum) are more expensive.
- Form: Sheet, plate, bar, tube, etc., all have different processing costs.
- Quantity: Buying in bulk usually gets you a better price per unit.
- Surface Finish: A highly polished mirror finish will cost more than a standard mill finish.
- Market Conditions: Global supply and demand for raw materials (like nickel and chromium) play a huge role.
- Production Costs & Supplier Markups.
The best way to get an accurate price for your specific needs is to contact stainless steel suppliers or distributors directly and request a quote. Some industry publications also track general market trends for stainless steel.
- F. Will stainless steel eventually rust? Under what conditions?
While incredibly resistant, stainless steel is not 100% “rust-proof” in all conceivable situations. It can corrode if:
- The wrong grade is used for the environment: A basic 304 grade might struggle in a harsh marine environment with lots of salt spray, where a 316L or a duplex grade would perform much better.
- The passive layer is continuously damaged and can’t reform: For example, in very low-oxygen environments or if constantly abraded.
- Exposure to aggressive chemicals: Strong chlorides (like bleach), certain acids (like hydrochloric acid), or prolonged contact with carbon steel (which can cause galvanic corrosion) can be problematic for some grades.
But for most everyday applications, and when the correct grade is chosen, stainless steel will live up to its “stainless” name for a very, very long time!
- G. Is all stainless steel magnetic? Which types are/aren’t?
Not all of it! This is a common point of confusion. Here’s the scoop:
- Austenitic Stainless Steels (like 304, 316): In their fully softened (annealed) state, these are generally NON-MAGNETIC. This is due to their face-centered cubic (FCC) crystal structure. However, if you cold work them (bend, stretch, or machine them heavily), they can become slightly magnetic.
- Ferritic Stainless Steels (like 430, 409): These are MAGNETIC, due to their body-centered cubic (BCC) crystal structure.
- Martensitic Stainless Steels (like 410, 420): These are also MAGNETIC, due to their body-centered tetragonal (BCT) crystal structure.
- Duplex Stainless Steels (like 2205): Because they have a mix of austenite and ferrite in their structure, they are MAGNETIC.
- Precipitation Hardening (PH) Stainless Steels: These are usually MAGNETIC.
So, if a magnet sticks to it, it’s likely a ferritic, martensitic, or duplex grade. If it doesn’t (or only very weakly), it’s probably an austenitic grade.
- H. What is 18/8 stainless steel?
- As we touched on earlier, “18/8 stainless steel” is a common way to describe an austenitic stainless steel that contains approximately 18% chromium and 8% nickel. The most common grade that fits this description is Type 304.
- I. What is 18/10 stainless steel?
- “18/10 stainless steel” typically refers to an austenitic stainless steel with approximately 18% chromium and 10% nickel. The extra 2% nickel (compared to 18/8) generally gives it slightly better corrosion resistance and often a brighter finish. It’s often seen as a premium choice for cutlery and cookware. This can include high-quality Type 304 or even some Type 316 grades.
- J. What is stainless steel soap and how does it work?
- This is a neat one! Stainless steel “soap” is simply a piece of stainless steel, often shaped like a bar of soap. It’s used by rubbing it in your hands under running water, much like regular soap, to remove strong odors like garlic, onion, or fish.
The exact science isn’t fully understood, but it’s thought that the stainless steel binds with the sulfur compounds that cause these odors, neutralizing them or lifting them from your skin. It doesn’t lather or clean dirt like traditional soap, but it’s surprisingly effective for those stubborn kitchen smells! - K. What toxic substances are released when welding stainless steel?
- Welding stainless steel can produce fumes containing various substances, some of which can be harmful. One of the main concerns is hexavalent chromium (Cr(VI)). Chromium itself is a key ingredient in stainless steel, but when it’s heated to high temperatures during welding, some of it can convert into the hexavalent form, which is a known carcinogen and can cause respiratory problems.
Other substances in welding fumes can include nickel, manganese, and iron oxides. That’s why it’s absolutely critical for welders to use proper ventilation (like fume extractors) and wear appropriate personal protective equipment (PPE), including respirators designed for welding fumes. Safety first! - L. What is the lifespan of stainless steel products?
This is like asking “how long is a piece of string?” – it really depends! The lifespan of a stainless steel product can range from many decades to even centuries, depending on:
- The Grade of Stainless Steel: Higher alloyed grades designed for aggressive environments will last longer in those conditions.
- The Application and Environment: A stainless steel sink in a home kitchen will likely last much longer than a component in a harsh chemical plant.
- Maintenance: Regular cleaning and proper care can significantly extend its life.
- Design and Fabrication Quality: A well-designed and properly fabricated part will perform better.
But generally, one of the major advantages of stainless steel is its exceptional durability and long service life, often outlasting many other materials.
- M. How is stainless steel grade determined?
- Stainless steel grades are primarily determined by their chemical composition – the precise percentages of chromium, nickel, molybdenum, carbon, and other alloying elements. This recipe then dictates the steel’s microstructure (austenitic, ferritic, etc.) and its resulting mechanical and physical properties (like strength, hardness, and corrosion resistance).
Industry standards, like those from ASTM, AISI, and EN, provide detailed specifications for each grade, outlining the acceptable ranges for chemical composition and expected performance characteristics. Manufacturers then produce steel to meet these specific grade requirements through careful control of the melting and alloying processes. - N. What’s better: stainless steel or sterling silver for jewelry/cutlery?
Both have their place, but they are very different!
- Stainless Steel:
- Pros: Much more durable, highly resistant to tarnish and corrosion, requires less maintenance, much more affordable.
- Cons: Can sometimes be seen as less “prestigious” than silver for very fine items.
- Sterling Silver (92.5% silver, 7.5% other metals, usually copper):
- Pros: Traditionally valued for its beauty, luster, and prestige, especially for fine jewelry and heirloom cutlery.
- Cons: Much softer and less durable than stainless steel (scratches and dents more easily), tarnishes over time and requires regular polishing, more expensive.
For everyday cutlery and fashion jewelry where durability and ease of care are important, stainless steel is often a practical and excellent choice. For special occasion silverware or fine jewelry where tradition and intrinsic value are key, sterling silver is often preferred.
- Stainless Steel:
Hopefully, these answers help clear up some common questions. Stainless steel is a deep topic, but understanding these basics goes a long way!
XXI. Conclusion: The Enduring Value and Future of Stainless Steel

What a journey we’ve been on! From its humble beginnings as a “rustless” curiosity to its current status as a cornerstone of modern industry and daily life, stainless steel has proven itself time and time again. We’ve seen how its remarkable blend of corrosion resistance, strength, versatility, and aesthetic appeal makes it an indispensable material across countless applications.
Whether it’s the gleaming facade of a skyscraper, the life-saving precision of a surgical tool, the robust machinery in a factory, or the humble spoon in your kitchen drawer, stainless steel quietly and reliably performs its duties. Its ability to withstand harsh environments, maintain hygiene, and endure for decades makes it a truly sustainable and cost-effective choice in the long run.
Understanding the different families – austenitic, ferritic, martensitic, duplex, and precipitation hardening – and the nuances of specific grades allows us to harness the full potential of this incredible alloy. As we’ve explored, choosing the right type of stainless steel, with the correct composition and finish for the intended application, is paramount to its success. It’s not just “steel”; it’s a highly engineered material designed for performance.
Looking ahead, the future of stainless steel is brighter than ever. With ongoing research leading to even more advanced alloys, innovative manufacturing techniques like 3D printing, and a growing global emphasis on sustainable materials (and stainless steel is highly recyclable!), its role will only continue to expand. From renewable energy technologies to cutting-edge medical devices, stainless steel will be there, pushing the boundaries of what’s possible.
It has been a privilege to share my insights and experiences with this remarkable material. I hope this guide has not only informed you but also sparked a new appreciation for the “superhero of metals” that is stainless steel.
Have more questions about stainless steel or need guidance selecting the perfect grade for your next project?
Don’t hesitate to reach out to our team of experts. We’re passionate about stainless steel and ready to help you find the best solutions! Contact Us Today!




